Summary
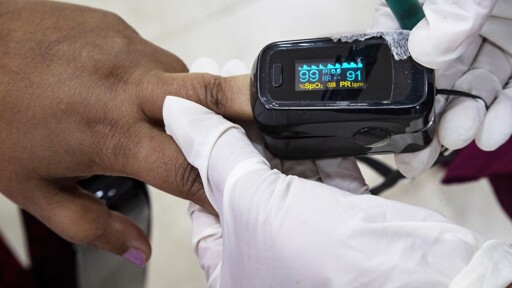
The FDA has proposed stricter testing requirements for pulse oximeters after studies revealed inaccuracies in readings for people with darker skin tones.
The draft guidelines call for clinical trials with at least 150 participants, including at least 25% with darker skin, and mandate multiple methods to measure skin pigmentation.
These standards apply to professional-grade oximeters in medical settings, excluding over-the-counter devices. Existing devices won’t be impacted unless modified.
Public feedback will be collected for 60 days before finalizing the rules to address racial biases in medical technology.
With thicker skin, too.
Those who work with their hands often have wildly inconsistent readings with pulse oximeters.
It’s a tool with limitations that must be accounted for
RFK Jr cancelling this: “We aren’t wasting precious resources that can go into anti-autistic ableism on my watch.”